Testing laboratories provide vital services to their customers who expect accurate results produced at appropriate time and at reasonable cost. However, when making measurements, there is always some level of inaccuracy. The challenge is to reduce the level of inaccuracy as much as possible, given the complexity of analytical testing laboratories that, like any type of complex system, exhibit an inherent variability. Therefore, the Quality Management System model (QMS), which looks at the entire system, is very important for assessing and controlling the laboratory complexity and achieving good performance.
According to the International Organization for Standardization (ISO), a quality management system can be defined as “coordinated activities to direct and control an organization with regard to quality”. QMS is a System for Managing the Quality of a product or process. Furthermore, QMS is a system for documenting the structure, procedures, responsibilities and processes needed for effective quality management. The QMS outlines how an organization will produce, document, control and deliver a product or service possessing customer perceived value.
The entire set of operations that occur in testing is usually called “path of workflow”: it begins with the sample collection and ends in reporting. The concept of the path of workflow is a key to the QM and must be considered when developing quality practices. For instance, a report that is delayed or lost can negate all the effort of performing the test well.
For this reason promoting a culture of quality should be an everyday effort and laboratories must remember that many factors should be addressed in order to assure quality.
Some of these factors include:
- the laboratory environment
- communications
- record keeping
- competent staff
- good-quality reagents and equipment
- constant evaluation
When all the laboratory procedures and processes are organized into an understandable and workable structure, the opportunity to ensure that all are appropriately managed is increased.
The QM presented here organizes all of the laboratory activities into 12 quality system essentials. These quality system essentials are a set of activities that must be addressed if overall laboratory quality improvement is to be achieved.
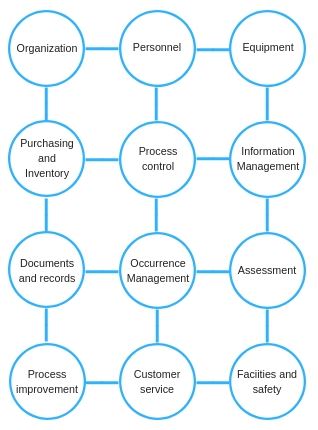
For the purpose of this discussion, one of these essentials is particularly important, i.e. Information Management.
Information Management is a system that incorporates all the processes needed for effectively managing data. Remember that data, and in particular test results, are the final product of the laboratory. For this reason, laboratory directors need to ensure that the laboratory has an effective information management system in place in order to achieve accessibility, accuracy, timeliness, security and privacy of information.
Eusoft.Lab LIMS can handle the steps of Acquisition – Processing – Archiving for all data generated by the laboratories. It can automate all key processes (sample acceptance, results entry, validation, generation of test reports). The flexibility of Eusoft.Lab allows the laboratory to computerize the management of its activities according to specific workflows for each process.
Please do not hesitate to contact us for further information: info@eusoft.co.uk




